Current Main Projects
Developmental Biology
Developmental biology is about the dynamic processes that play out at the cellular and tissue level to create an organism. Its three fundamental aspects are morphogenesis (what causes an organism to develop its shape), the control of cell growth, and cellular differentiation. Key to these are the mechanisms that underlie a cell's decision-making to determine its fate and specification. Recent developments in single-cell transcriptomics, proteomics and imaging have opened up exciting opportunities to probe these decision-making mechanisms in much deeper ways than previously possible.
Dynamical systems & Waddington landscapes
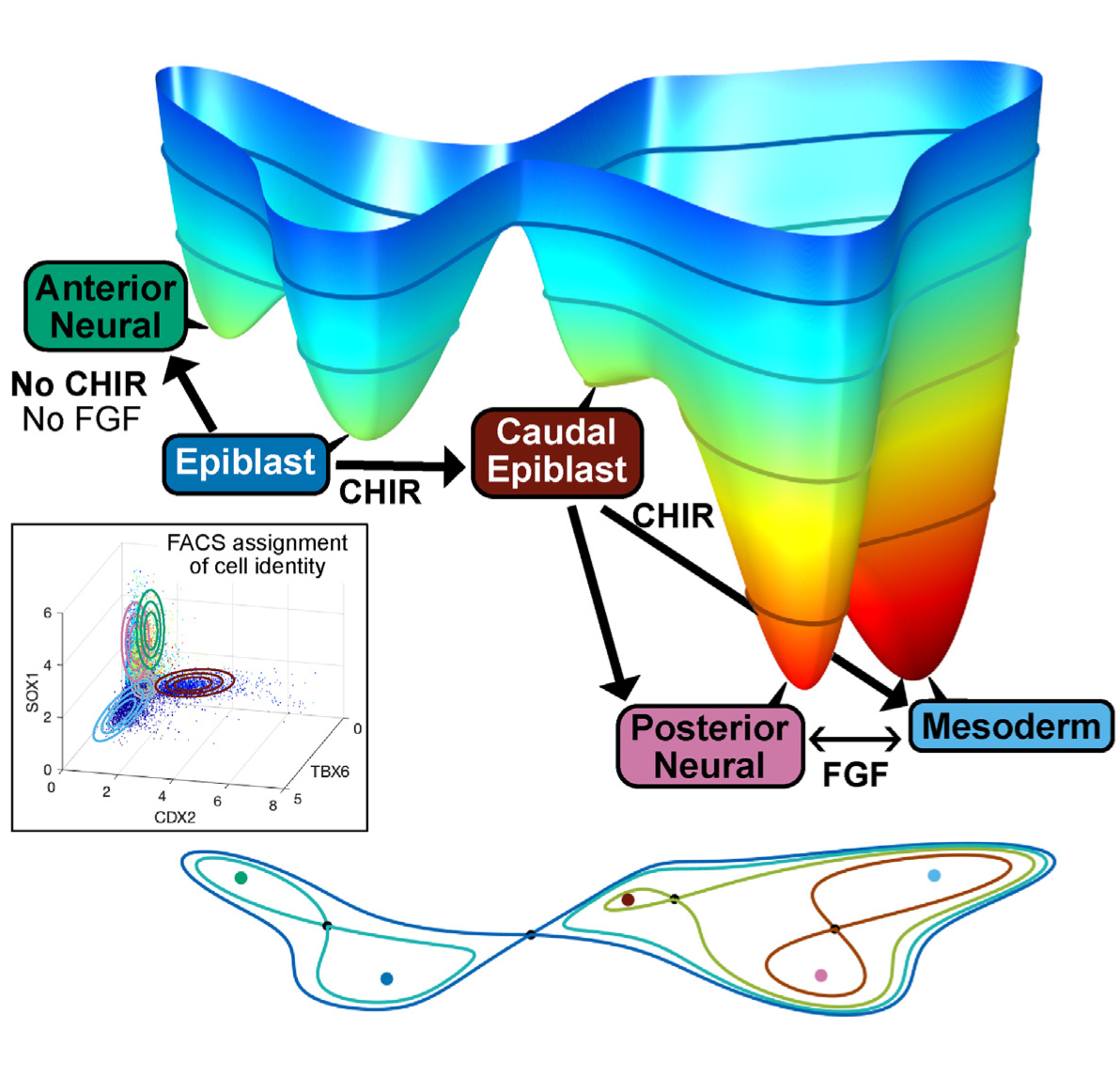
Developmental dynamics admits an intuitive topographical representation that can be formalised mathematically (C H Waddington, The Strategy of the Gene, London, 1957). The developmental path of a cell is conceived as a downhill flow in a landscape with height function that is itself shifting because of changes in the signals the cell receives. In this picture, cell fates are determined by which of the valleys the downhill trajectory descends to. Changing signals can cause the attractor in that valley to disappear (bifurcate) allowing a dynamically trapped cell to transition to a new fate.
Part of my work to to make this intuitive picture rigorous using the modern theory of dynamical systems. The dynamical system we are concerned with are those which describe relevant gene regulatory networks comprising transcription factors and intercellular signals. Under general conditions they have a gradient-like description which allows the link with landscapes.
Cell differentiation whereby cells change their state and develop specialised functions is at the heart of developmental biology. In our landscape picture it occurs when there is a bifurcation which destroys an attracting state allowing the cells that were trapped by that attractor to escape and transition to a new attractor which corresponds to a new fate. The developmental transitions produced by these bifurcations are caused by changes in the signals that the cells see and typically there is a relatively small number of signals. A key aim of our theory is a classification of such bifurcations as part of a classification of compact landscapes. Already, with the classification of landscapes in dimension 2 we find interesting examples highly relevant to stem cell differentiation.
Recent Papers
Dynamical landscapes of cell fate decisions
M. Sáez, J. Briscoe and D. A. Rand
Interface Focus 12: 20220002. https://doi.org/10.1098/rsfs.2022.0002
A quantitative landscape of cell fate transitions identifies principles of cellular decision-making.
M. Sáez, R. Blassberg, E. Camacho-Aguilar, E. D. Siggia, D. A. Rand, J. Briscoe
Cell Systems 12:1–17 https://doi.org/10.1016/j.cels.2021.08.013
Geometry of Gene Regulatory Dynamics.
David A. Rand, Archishman Raju, Meritxell Sáez, Francis Corson, and Eric D. Siggia.
Proceedings of the National Academy of Sciences of the USA. (2021) 118:38:e2109729118 https://doi.org/10.1073/pnas.2109729118
Quantifying cell transitions in C. elegans with data-fitted landscape models.
Elena Camacho-Aguilar , Aryeh Warmflash, David A. Rand
PLoS Computational Biology, 17(6), p.e1009034
Collaborators
James Briscoe, Eric Siggia, Marine Fontaine, Meritxell Sáez, Francis Corson, Elena Camacho-Aguilar, Aryeh Warmflash, Ina Sonnen.
Circadian clocks
The circadian clock adapts organisms to the environmental day-night cycle both behaviorally and physiologically. In animals, not only are complex behaviors such as sleep and mood governed by this oscillator, but also different body functions such as digestion, circulation, and respiration. The basic mechanism of this clock is cell-autonomous in all studied species possessing a circadian clock i.e. each cell contains a network of genes and proteins that can generate an oscillator with a period of approximately 24 hours. These oscillators can, in turn be entrained, to the relevant 24-hour environmental day-night cycle.
My research in this area is at the moment focused on the use of our algorithm TimeTeller to provide a circadian rhythm biomarker for clock functionality More generally, I am very much concerned with understanding the design principles of circadian clocks. What are their roles and how is this reflected in their structure? I would add that since the clock is a dynamical system with a very complex molecular structure and since the role of the clock is clearly multifacited, this cannot be answered without the use of mathematics. My collaborators are listed here.
A biomarker for circadian rhythms
The circadian timing system controls several critical molecular pathways for cancer processes and treatment effects. While in the cells of most healthy tissues the cell cycle is gated or phase-locked by the circadian clock, cancer cells often escape this control and display altered molecular clocks. Dysregulation of clock genes promotes tumorigenesis through mechanisms involving the cell cycle, DNA damage, and metabolism. Moreover, the circadian clock rhythmically controls many molecular pathways which are responsible for large time-of-day dependent changes in drug toxicity and efficacy, whilst clock gene expression is correlated to anti-cancer drug sensitivity in cancer cell lines. Altered expression of clock genes is associated with key oncogenic pathways and patient survival, and the correlation between clock genes and other genes is altered in cancerous tissues.
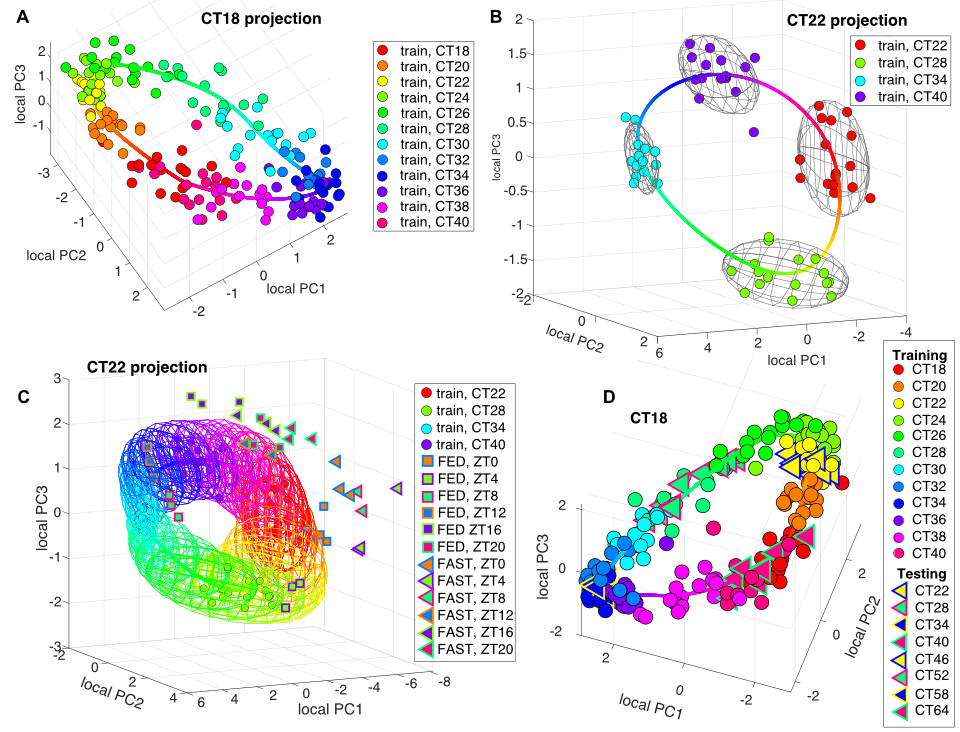
We have developed a machine-learning approach to measuring circadian clock functionality from the expression levels of 10-16 key genes in a single tissue sample. The availability of our single sample algorithm TimeTeller potentially allows the determination of clock dysfunction in an individual patient along the course of his or her disease and treatments, the study of its relations with patient outcomes, and the use of such measurements in clinical practice.
To illustrate its utility we applied TimeTeller to breast cancer where previous studies have highlighted the relevance of circadian clocks for carcinogenesis and treatment effects but where no simple method would currently allow its measurement in daily oncology practice. We find a strong association between overall and disease-free survival and our measure of clock dysfunction in breast cancer patients.
Recent papers
TimeTeller: A tool to probe the circadian clock as a multigene dynamical system.
Vlachou D, Veretennikova M, Usselmann L, Vasilyev V, Ott S, Bjarnason GA, Levi F, & Rand, D.
PLoS Comput Biol 20(2): e1011779. (2024) https://doi.org/10.1371/journal.pcbi.1011779
Collaborators
Current: Frances Levi, Robert Dallmann, Sylvie Giacchetti, Vadim Vasilyev, Past: Denise Vlachou, Maria Veretennikova, Laura Usselmann, Vadim Vasilyev V, Ott S, Georg Bjarnason,
Global Bifurcation Theory
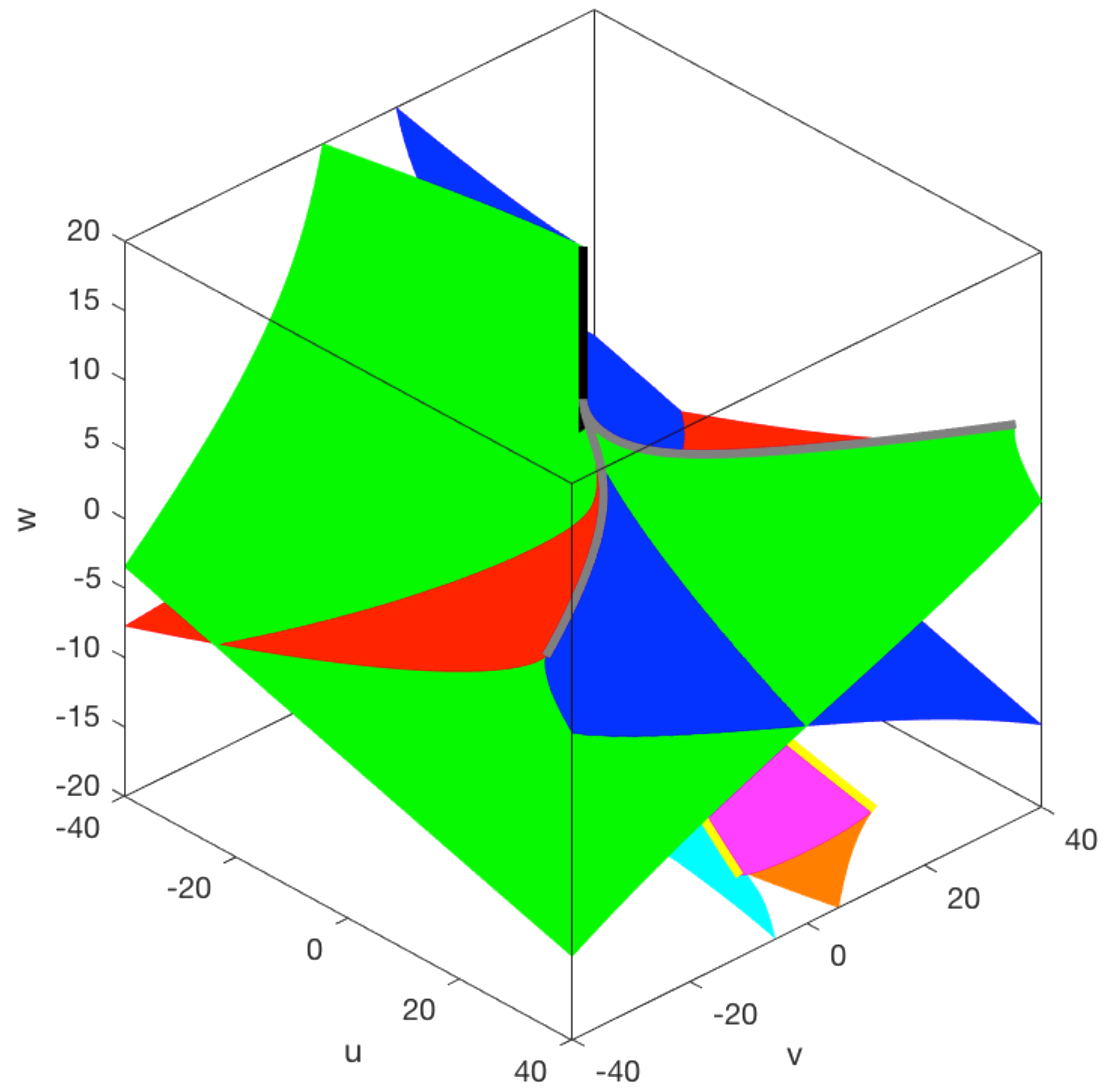
The aim here is to study global structures and prove global results that are about systems rather than local ones about germs. An example is a theorem that says that if a parameterised system with a finite number of restpoints and periodic orbits and a simply connected 2d parameter space has just two opposed fold points on its boundary and no others, then there is either a fold-Hopf point or an odd number of cusps in the interior of the parameter space.
The figure on the left is the bifurcation set for the compact elliptic umbilic. It has been used to describe the landscape underlying a key part of the developmental process creating the worm vulva (Corson & Siggia). Thanks to Meritxell Saez for analysing and plotting the figure.
Recent Preprint
Bistable boundary conditions implying codimension 2 bifurcations
Sáez, M & Rand, D.
Preprint
Collaborators
Marine Fontaine, Meri Saez